Structure & Properties of Hydrogen-based DRI Compared with Natural Gas-Based Reduction

Introduction
The mechanical properties of direct reduced iron (DRI), such as swelling behavior and strength are influenced by reducing gas composition. Zhao et al. reported that the higher carbon monoxide (CO) concentration is in reducing gases, the more serious the swelling that occurs1. Mizutani et al. examined the relationship of the reduction disintegration tendency and the reduction mode and concluded that the topochemical reduction in hydrogen (H2) and CO mixture resulted in worse disintegration behavior than the homogeneous reduction under pure H2 atmosphere2. These studies indicate that there is less possibility of swelling and disintegration when DRI is produced under H2.
The use of pure hydrogen (H2) as direct reduction reducing gas gives rise to the question whether the as-produced cold DRI (CDRI) will reach the same targeted parameters, such as product strength, when compared to CDRI produced from reformed natural gas (NG).
Investigations were conducted at the Midrex Research & Development Technology Center to examine and compare the effects of gas composition on the apparent density, porosity, reduction swelling, cold crush strength, and fines generation of carburized and non-carburized CDRI when using either H2 or reformed NG as the reducing gas. We determined that the structure and physical properties of CDRI are influenced by the reducing gas composition and subsequently may affect plant operation or cause materials loss during processing and transportation.
Testing & Calculating Methodology
Five direct reduction grade-pellets, with total Fe ranging between 67.6% and 68.3%, were selected and subjected to two modified ISO 11258 reducibility tests. Two types of reducing gas were employed for the reducibility tests (labeled Standard NG and Standard H2). The gas compositions of the Standard NG and Standard H2 reducing gases are shown in Table 1.

TABLE I.
Gas Composition Standard NG and Standard H2 reducing gas
The pellets reduced under Standard NG reducing gas were further carburized to achieve around 2% carbon level to mimic industrial DRI. After the pellets completed the reduction process under Standard NG conditions, the NG-reduced DRI was immediately subjected to 30 minutes of cooling under nitrogen (N2) flow to a temperature of 700°C, followed by carburization under a continuous flow of a mixture of H2 and CH4 (methane) gas, maintained at 700°C during the carburization process.
The physical properties of the H2-DRI and the carburized NG-DRI, such as apparent density, porosity, volume expansion, cold crushing strength, and tumble strength were measured using methods developed in-house. The reduction degree of the pellets at any given time was determined by weight loss of the samples during reduction based on reduction degree at a set time, weight change in load cell at a set time during the reduction test, and total weight change of sample after the completion of the reduction test.
The reduction degree of the DRI was determined by measuring the concentration of total Fe (%T.Fe), metallic Fe (%M.Fe), and FeO (%FeO) via chemical analysis.
The carburizing percentage of NG-DRI was determined by weight gain of the samples during carburization. The volume expansion ratio of DRI was determined with a series of equations that considered volume of sample, sample weight after reduction and carburization, weight of oxygen combined with iron as Fe2O3 and Fe3O4 in oxide pellet, weight of oxygen removed by reduction, sample weight after reduction, apparent density of sample, and oxygen concentration of the oxide pellet.
Results
Chemical analysis and reduction curves of oxide pellets and DRI
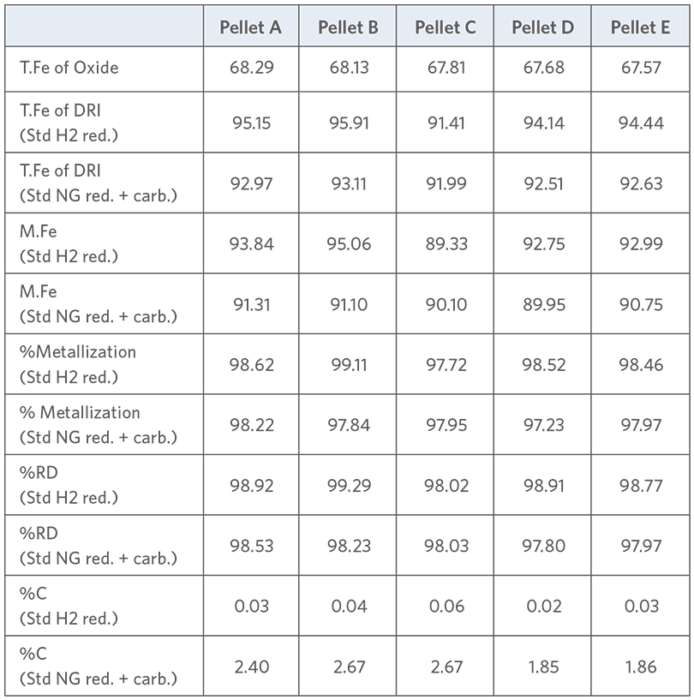
TABLE II.
Typical DR-grade oxide pellets characteristics
Table II shows the chemical analysis of the five selected pellets in their initial oxide state after Standard H2 reduction and after Standard NG reduction followed by carburization, respectively.0
The reduction curves of the five oxide pellets that were reduced under Standard H2 and Standard NG reduction conditions are shown in Figure 1A and Figure 1B.
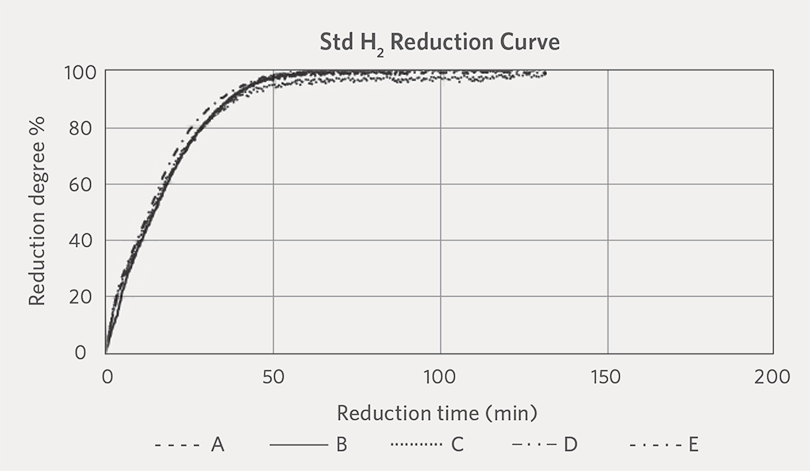
FIGURE 1A.
Reduction curves under Standard H2 conditions at 800°C
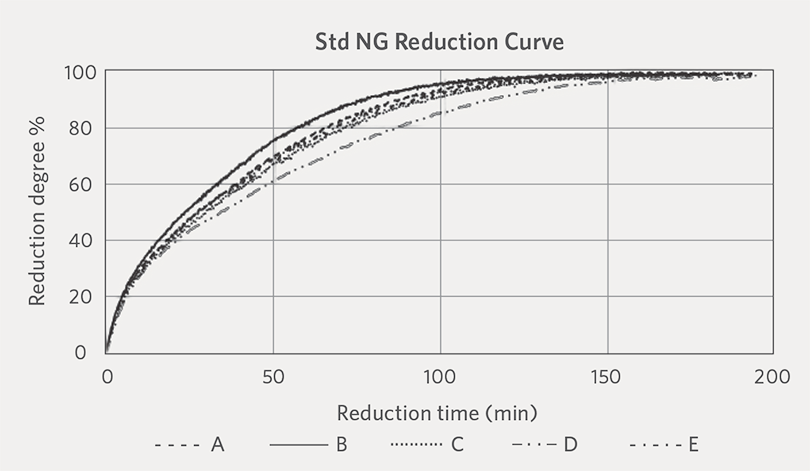
FIGURE 1B.
Reduction curves under Standard NG condition at 800°C
The reduction curves indicate that under the same reduction temperature (800°C), the reduction rate (i.e., for achieving a given reduction degree) was the fastest for the pellets reduced under the Standard H2 reduction condition. We also observed that there was not much difference in the reduction behavior of the different oxide pellets when they were reduced under the Standard H2 condition. On the other hand, the reduction rate varied among the different oxide pellets when they were subjected to Standard NG reduction condition. The reason for the different reduction behaviors between the oxide pellets and reduction conditions is not clear, as there are many factors that can affect the reduction curve of oxide pellet including pellet size distribution, iron oxide grain size distribution, size of pores, pores complexity inside the pellet, slag phase, bonding strength of oxide grains, and pelletizing temperature.
Apparent density and porosity comparison
Figure 2 and Figure 3 show the average apparent density and porosity of oxide pellets, H2-DRI, and carburized NG-DRI. There is no noticeable difference between the H2-DRI and NG-DRI.
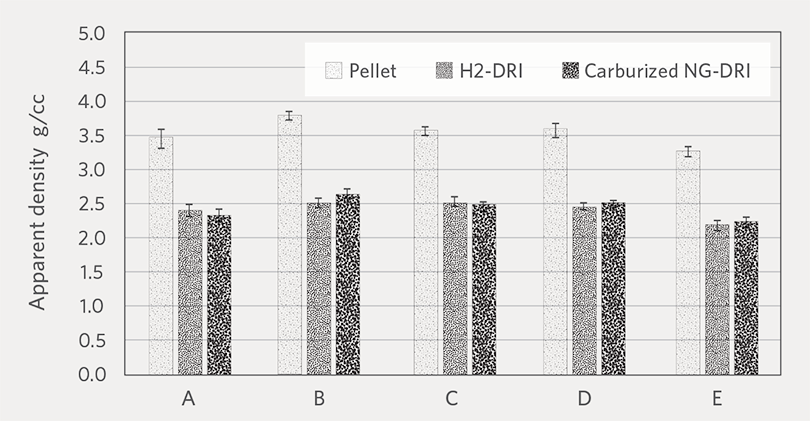
FIGURE 2.
Apparent density of oxide pellet, H2-DRI, and carburized NG-DRI
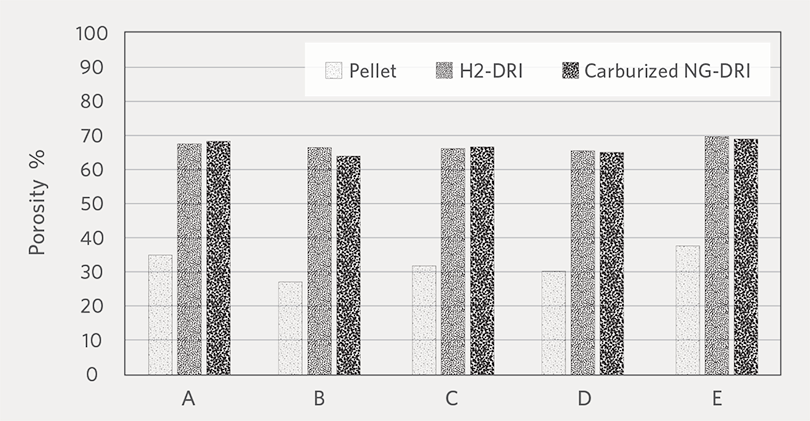
FIGURE 3.
Porosity of oxide pellet, H2-DRI and carburized NG-DRI
Figure 4 shows the relationship between the porosity of oxide pellets and DRI. As can be seen in Figure 4, the porosity of DRI trends with the porosity of the respective oxide pellets and this suggests that the porosity change during the reduction process, or the final porosity of the reduced pellets, is mainly governed by the pre-reduced oxide pellets.
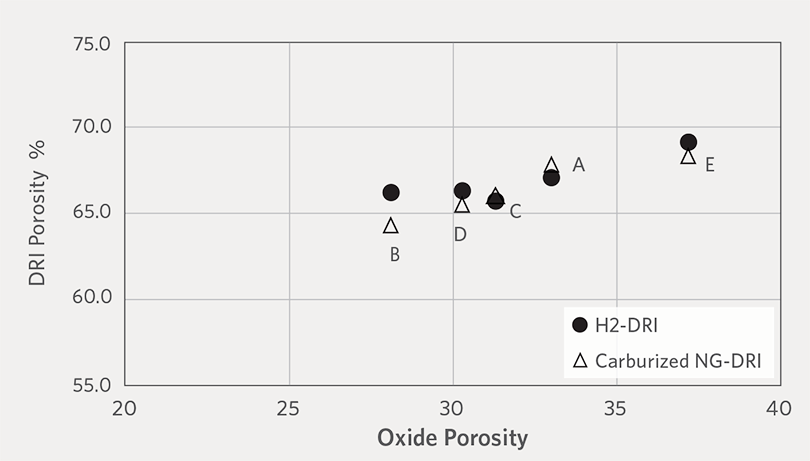
FIGURE 4.
Comparison between porosity of oxide pellet, H2-DRI and carburized NG-DRI
Volume expansion comparison
Figure 5 shows the volume expansion comparison between H2-DRI and carburized NG-DRI of each pellet brand. For all oxide pellet brands, hydrogen reduction produced comparable or smaller volume expansion than reformed natural gas reduction.
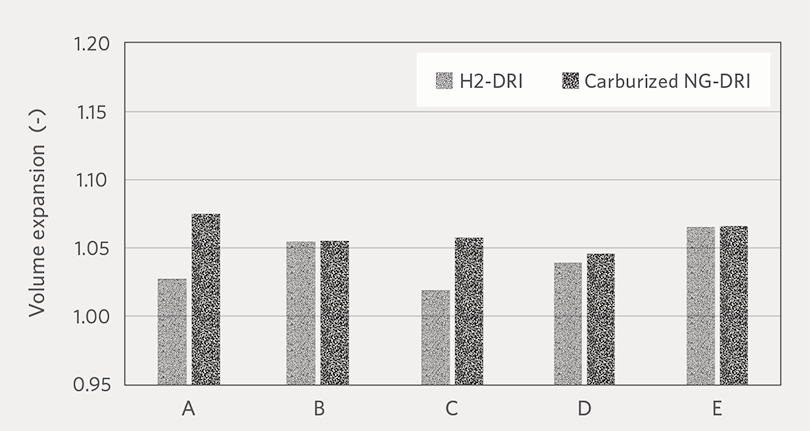
FIGURE 5.
Volume expansion comparison between H2-DRI and carburized NG-DRI

Cold Crushing Strength (CCS comparison)
Figure 6 shows the average CCS (kgf/ piece) of H2-DRI and NG-DRI. For all pellet brands, H2-DRI has slightly higher CCS than NG-DRI. Based on this result, we can expect CDRI produced from H2 reduction to have less cracks or breakage during handling or transportation compared to NG-CDRI.
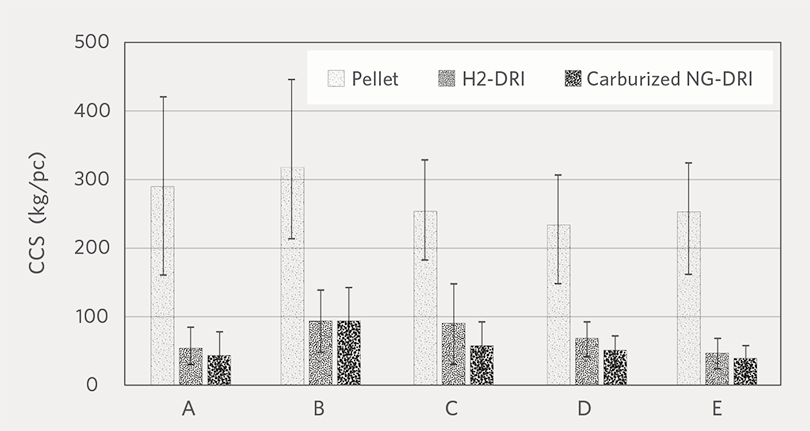
FIGURE 6.
CCS comparison between oxide pellet, H2-DRI and carburized NG-DRI
Tumble Strength (CCS comparison)
Figure 7A and Figure 7B show the average weight ratio of fines at -3.35 mm and -1.0 mm generated H2-DRI and NG-DRI, respectively. For all the pellet brands, H2-DRI
generated less fines compared to carburized NG-DRI. Based on this result, we can expect that cold H2-DRI would generate less fines during handling than NG-DRI. One possible reason for the higher fines generation by carburized NG-DRI could be that the Fe3C (iron carbide) phase has less resistance to abrasion than metallic iron.
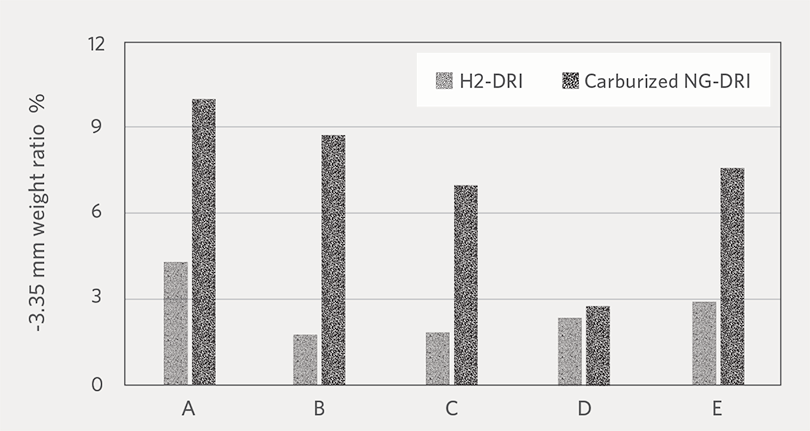
FIGURE 7A.
Weight ratio of -3.35 mm fines generated by H2-DRI and carburized NG-DRI
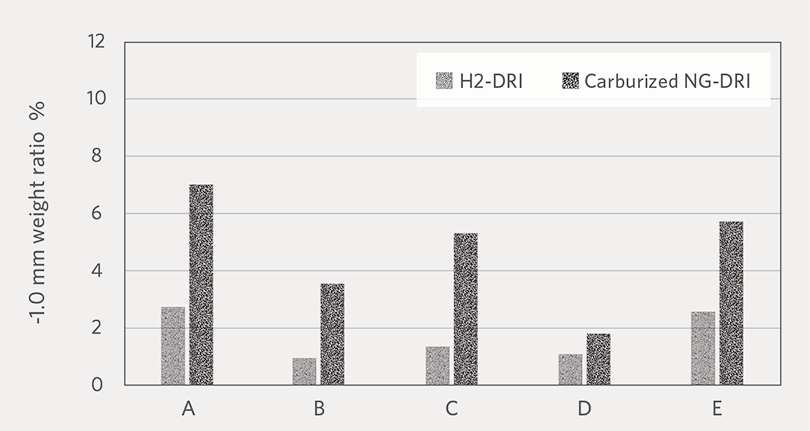
FIGURE 7B.
Weight ratio of -1.0 mm fines generated by H2-DRI and carburized NG-DRI
Discussion
Effect of reduction gas composition on physical properties
To better understand the effect of H2 reduction on the physical properties of DRI, the CCS and weight ratio of fines results were compared with respect to DRI porosity. Figure 8A and Figure 8B show the relationship between porosity and CCS of H2-DRI and carburized NG-DRI, respectively. The result shows that for a given porosity, the CCS of H2-DRI is stronger than carburized NG-DRI and the compression strength decreases with increasing porosity.
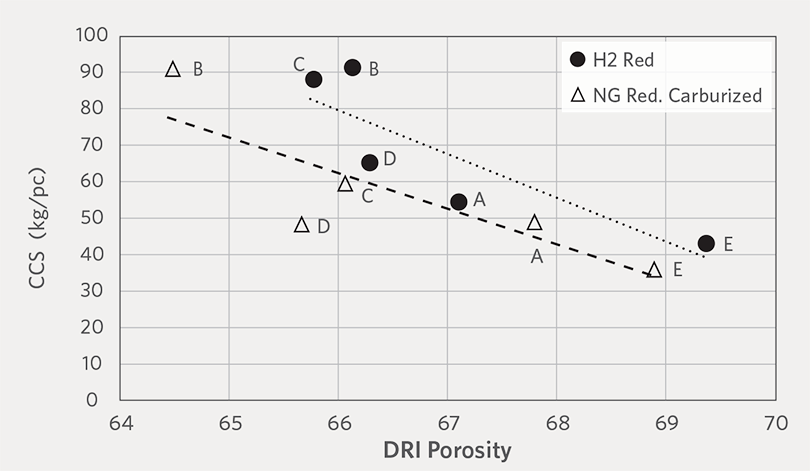
FIGURE 8A.
Relationship between porosity and CCS of H2-DRI and carburized NG-DRI
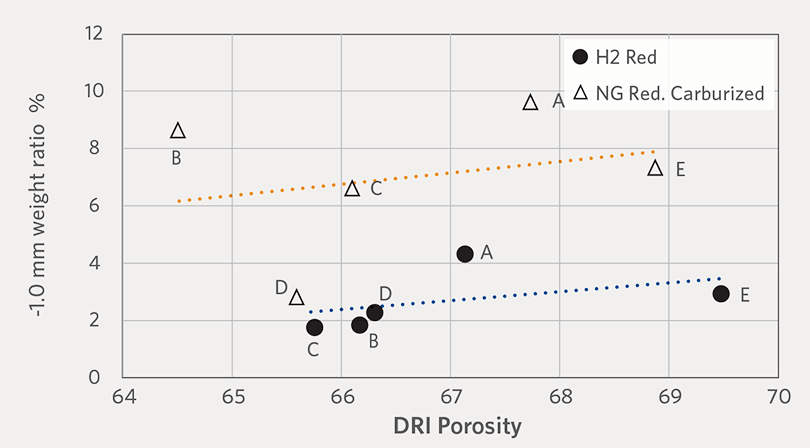
FIGURE 8B.
Relationship between fines generated and CCS of H2-DRI and carburized NG-DRI
Furthermore, Figure 9 shows the relationship between porosity and fines generation of H2-DRI and carburized NG-DRI. Both conditions have exhibited linear relationship of fines generation over DRI porosity. However, H2 reduction produced lower amount of fines generation than carburized NG-DRI.
Thus, we can conclude that hydrogen reduction allows higher strength and lower fines generation for CDRI than NG reduction with carburization. Among the different DR-grade pellet brands, oxide pellet porosity is key for determining CDRI physical properties.
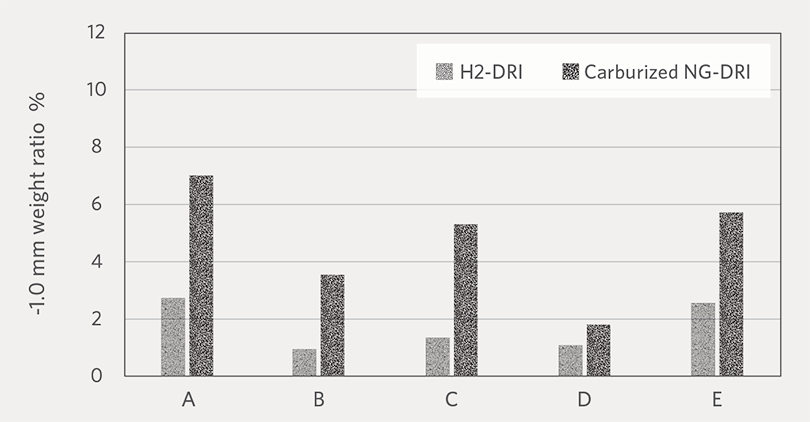
FIGURE 9.
Weight ratio of -1 mm fines generated by H2-DRI and carburized NG-DRI
Conclusions
A detailed study and comparison of physical properties of H2-DRI and carburized NG-DRI produced from five DR-grade oxide pellets was conducted. There was no apparent difference in apparent density, porosity, and CCS between H2-DRI and carburized NG-DRI. Fines generation by H2 reduction is comparable or lower than that by NG reduction. Specifically, H2 reduction had lower<1mm fines generation than NG reduction. CCS and fines generation correlated with DRI porosity. The descending order of pellet brands for DRI porosity is similar with that for oxide pellet porosity. Thus, oxide porosity is a key factor determining the physical properties of CDRI.

References:
- Zhao, Z., Tang, J., Chu, M., Wang X., Zheng, A., Wang, X., Li, Y., Direct reduction swelling behavior of pellets in hydrogen-based shaft furnaces under typical atmospheres, International Journal of Minerals, Metallurgy and Materials, Vol. 29, 2022, pp. 1891-1900.
- Mizutani, M., Nishimura, T., Orimoto, T., Higuchi, K., Nomura, S., Saito, K., Kasai, E., Influence of Reducing Gas Composition on Disintegration Behavior of Iron Ore Agglomerates, ISIJ International, Vol.57, 2017, pp. 1499-1508.
This article was developed from the paper titled, “Comparison of the Morphology and Physical Properties of CDRI Following Hydrogen and Natural Gas-Based Reduction and Carburization,” presented at AISTech, May 5-8, 2025, in Nashville, TN, USA, by Dr. Pei Yoong Koh of Midrex Technologies, Inc. and Dr. Kentaro Urata and Takayuki Koyama of Kobe Steel Limited.




