Energy Transition in the European Steel Industry – Reality Not Exception

INTRODUCTION
The German Steel Industry sent an open letter to the policy makers in Berlin and Brussels in mid-2017, hoping to receive some relaxation with regard to Emission Trading Cost and final CO2 emission targets, but to no avail. This was a turning point for the industry. Together with the acceptance of the reality of climate change, it became obvious that the time for CO2 emission reduction measures has come. Today every steel producer in Europe has made commitments to reduce its CO2 footprint and each of the programs includes direct reduction plants and the use of renewable hydrogen as the reducing agent.
 The steel industry, especially traditional ironmaking, is among the largest contributors of greenhouse gases emissions – in the range of 7-9% of total emissions – because of its significant reliance on coal. BF/BOF emissions can be 1.6-2.0 kg CO2/ kg steel depending on the technologies used. The natural gas-based MIDREX® Process paired with an EAF has the lowest CO2 emissions of any commercially proven steelmaking route using virgin iron ore at 1.1 – 1.2 kg CO2/kg steel. By adding a CO2 removal system, the MIDREX Process can lower CO2 emissions even further to less than 1/3 of the emissions from the BF/BOF route if CO2 can be stored and/or used.
The steel industry, especially traditional ironmaking, is among the largest contributors of greenhouse gases emissions – in the range of 7-9% of total emissions – because of its significant reliance on coal. BF/BOF emissions can be 1.6-2.0 kg CO2/ kg steel depending on the technologies used. The natural gas-based MIDREX® Process paired with an EAF has the lowest CO2 emissions of any commercially proven steelmaking route using virgin iron ore at 1.1 – 1.2 kg CO2/kg steel. By adding a CO2 removal system, the MIDREX Process can lower CO2 emissions even further to less than 1/3 of the emissions from the BF/BOF route if CO2 can be stored and/or used.
Yet, there is even more room for lower emissions through use of hydrogen as a fuel and chemical reactant in the MIDREX Process. The ultimate method for reducing the steel industry’s CO2 footprint is the use of ‘green’ hydrogen produced from renewable energy for DRI production.
‘GREEN’ HYDROGEN
Developing efficient electrolyser technology to maturity is a precondition for successful energy transition. In early 2019, Paul Wurth acquired a 20% participation in Sunfire, a leading developer of steam electrolysers for the production of renewable, low-cost industrial hydrogen. The motivation was to actively support and develop fossil-free ironmaking, a critical step in hydrogen-based steel production.
Electrolysis, which uses electricity to split water into hydrogen and oxygen, is a promising technology for hydrogen production on an industrial scale. Water electrolysis accounts for 4% of the global hydrogen production. Since the hydrogen molecules come from water and not hydrocarbons, it may be considered ‘green.’ However, there are three constraints for its industrial use: 1) in most countries, electricity is generated primarily with fossil fuels so there remains a large overall CO2 footprint, 2) hydrogen from electrolysis is not currently available in the volumes required for large industrial users like steelmaking and 3) the cost of hydrogen is too high for many applications at prevailing electricity prices.
There are several technologies at various levels of technical readiness to produce hydrogen from water. One of the most promising of these is solid oxide electrolysis cell (SOEC).
These cells work at high temperature (around 800°C). Sunfire ́s electrolysers require 26-28% less electricity, compared to alternative technologies, meaning that 35-40% less renewable energy is necessary for the same H2 output. Condition to this is the availability of low quality steam (e.g. 140 °C, 3 bar), which can be easily recovered from waste heat sources in metallurgical plants.
Another feature of Sunfire’s electrolysers is the capability of producing syngas (CO and H2) when being fed with CO2 and steam. This process is called co-electrolysis. Interesting applications are at hand for metallurgical plants, as well as other domains like synthetic fuel production, using CO2 from unavoidable CO2 sources.
A standard Sunfire-Hylink SOEC unit of the 3 MW class is shown in Figure 1.

FIGURE 1.
Standard Sunfire-Hylink SOEC Unit

TABLE I shows the technical details of Sunfire-Hylink SOEC process.
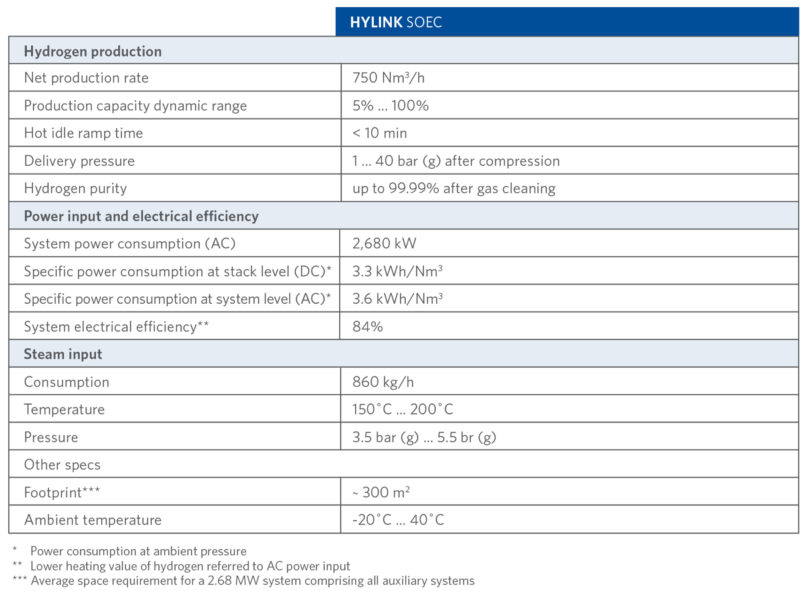
TABLE I.
Sunfire-Hylink SOEC Process Technical Details
Alkaline electrolysis, another technology for producing hydrogen from water, has been used at commercial scale for decades and is well proven. Recently Sunfire acquired Swiss Alkaline Electrolysis Company IHT, which designs and manufactures one of the most reliable and cost effective alkaline electrolysers in the market. IHT has a legacy of more than 70 years of experience worldwide and brings with it a reference plant capacity of 240 MW.
With its two differentiated electrolysis technologies – SOEC and Alkaline – Sunfire can offer the optimal solution for any hydrogen application and strengthens its position as a leading electrolysis technology provider in the green hydrogen revolution. Technical details of Sunfire-Hylink Alkaline process are shown in TABLE II.
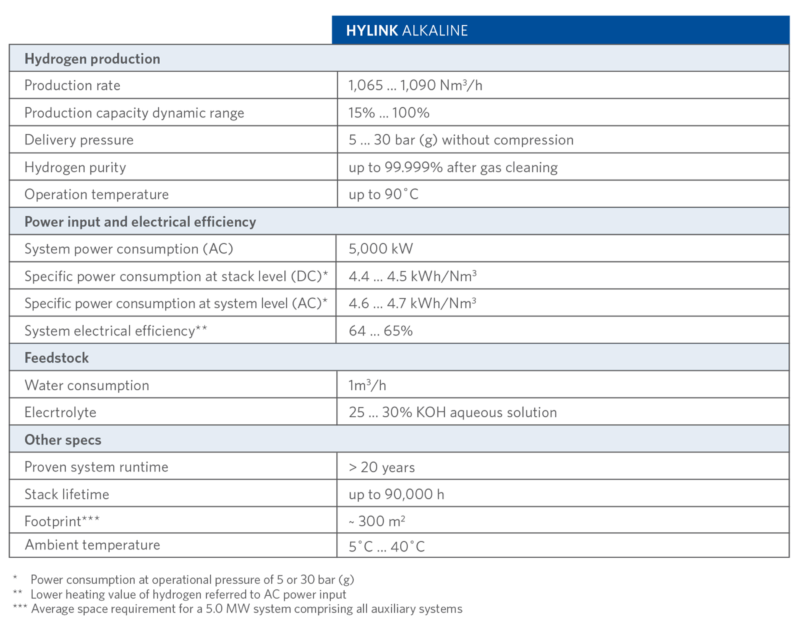
TABLE II.
Sunfire-Hylink Alkaline Process Details
A standard stack Sunfire-Hylink Alkaline unit of 5 MW scale is shown in Figure 2.

FIGURE 2.
Standard Stack Sunfire-Hylink Alkaline Unit
HYDROGEN FOR IRONMAKING
Industrial use of hydrogen, such as for producing iron, offers the advantage of a fixed location and large demand. Hydrogen can be generated on-site or supplied over-the-fence with significantly lower infrastructure cost per volume of gas. Steel mills also have the ability to integrate hydrogen generation with other utilities available on site, such as steam and other gases.
MIDREX Plants already use large amounts of hydrogen produced from reformed natural gas to convert iron oxide into DRI and can be adapted to accommodate more hydrogen as it becomes more available and economically priced. Currently, there are six MIDREX Modules that utilize gas made from coal, with hydrogen-to-CO ratios from 0.37 to 0.56. The FMO MIDREX Plant in Venezuela uses a steam reformer with an H2/CO ratio that has varied from 3.3 to 3.8 and has operated for decades. Thus, the MIDREX Process has successfully produced DRI with H2/CO corresponding to the range of 40% to 50% natural gas substitution with hydrogen.
The MIDREX Process has the flexibility to allow the addition of hydrogen to displace natural gas in the feed gas in the range of 0% to 100% with only minor equipment modifications. If hydrogen is abundant and economical at the onset of the project development, the process can be simplified into MIDREX H2™ (Figure 3).
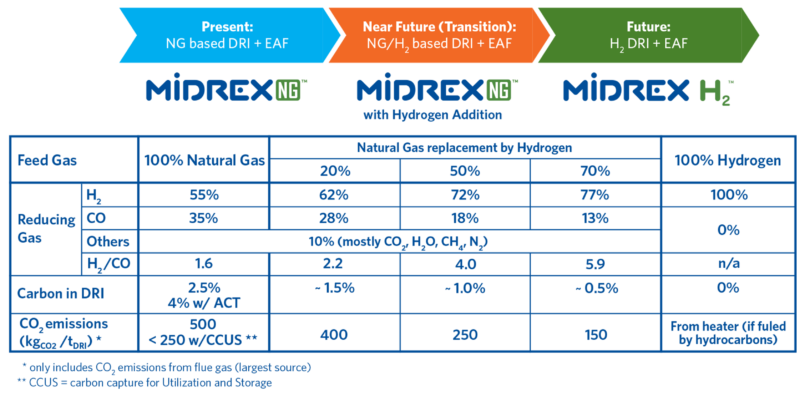
FIGURE 3.
MIDREX Process Using Hydrogen
CONCLUSION
Producing iron via the traditional blast furnace route is a large contributor to the emission of greenhouse gases, notably CO2. Mitigating CO2 emissions in the iron and steel industry is becoming critical worldwide, as the cost and social impact of CO2 emissions increases over time. The best possibility for reducing the steel industry’s CO2 footprint is the use of hydrogen as an energy source and reductant in the MIDREX Process for producing direct reduced iron (DRI).
Today, reduction of CO2 emissions by 50% (over BF/BOF) is achievable and well proven in the DRI-EAF steelmaking route. The MIDREX Process can accept ‘green’ hydrogen produced from water electrolysis as it becomes available and economical, which will further reduce CO2 emissions.
Paul Wurth has invested in Sunfire-Hylink, a German electrolyser technology company, in order to produce renewable, low-cost industrial hydrogen. This ‘green’ hydrogen can be used to support and develop fossil fuel-free ironmaking, and a Sunfire facility could provide the hydrogen for a MIDREX H2 Plant in a fossil fuel-free steel mill of the future (Figure 4).
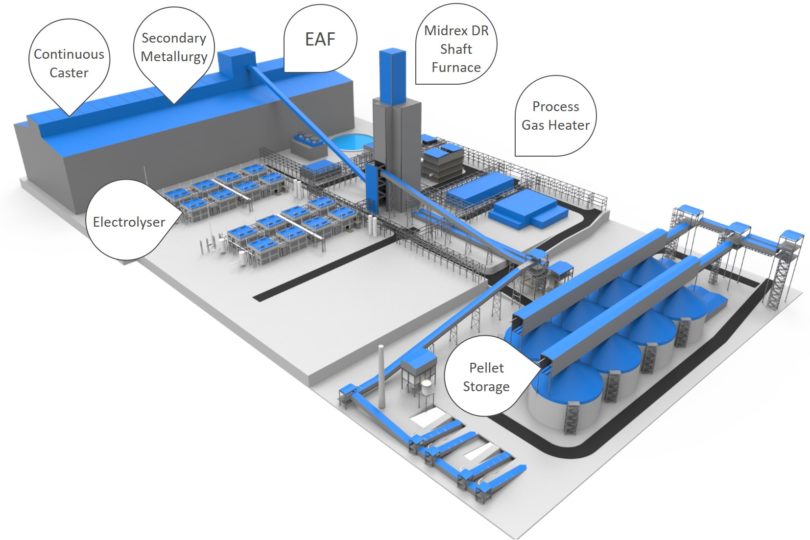
FIGURE 4.
Layout of Fossil-free Integrated DR-EAF Steel Mill with MIDREX H2 Plant Fueled by Sunfire Electrolysers



